PINCER: Monitoring medication safety throughout the COVID-19 pandemic using OpenSAFELY
- Authors:
-
Posted:
- Categories:
In this blog, Louis Fisher describes some of our recent work, now published in BMJ Medicine investigating the impact of the COVID-19 pandemic on safe prescribing using a set of quality assured indicators across 57 million patients’ records in England.
Medication errors and the PINCER intervention
Medication errors are a major source of potentially avoidable morbidity in primary care. The European Medicines Agency describes a medication error as “an unintended failure in drug treatment process that leads to, or has the potential to lead to, harm to the patient”. It is estimated there are around 240 million medication errors in England annually, with almost 40% of those occurring in primary care1.
A patient safety challenge launched by the WHO in 2017 aimed to “reduce severe avoidable medication related harm globally by 50% in the next 5 years”2. In response to this, PRIMIS, a specialist team of health informaticians from the University of Nottingham, who specialise in supporting behavioural change in general practice developed PINCER (pharmacist-led information technology intervention for medication errors). The PINCER intervention is a proven programme of activities for reducing hazardous prescribing in general practice. This involves training pharmacists to provide dedicated support to general practice by helping identify patients at risk of harm from medication using pre-specified and quality assured indicators3. This helps tackle the “Systems and practices of medication” element of the WHO patient safety challenge, shown in the figure below.

PINCER indicators throughout the COVID-19 pandemic
There are 13 PINCER indicators which show potentially unsafe prescribing of high risk medications that 1) can cause gastrointestinal bleeds, 2) are cautioned against in certain conditions (heart failure, asthma and chronic renal failure, 3) require blood test monitoring. Historically, only manual audits within a practice, or analyses of data downloaded from a group of practices have been possible. Using OpenSAFELY, in a single analysis executed within the secure environment inside electronic health record provider data centres, we have implemented the full set of PINCER indicators to describe any changes in adherence in over 95% of practices in England throughout the COVID-19 pandemic.
We showed that there was no evidence of increase in indicators of harm as captured by the PINCER indicators throughout the pandemic. This highlights that despite increased pressure on primary care throughout the pandemic, safe prescribing was appropriately prioritised as part of a dedicated and unified response by GP teams across the country.
You can find out more about the PINCER indicators and how they have changed throughout the COVID-19 pandemic in our manuscript, now published here. Below we’re going to focus specifically on the indicator associated with methotrexate, a high risk drug prescribed for autoimmune conditions such as rheumatoid arthritis, Crohn’s disease and psoriasis.
Methotrexate monitoring
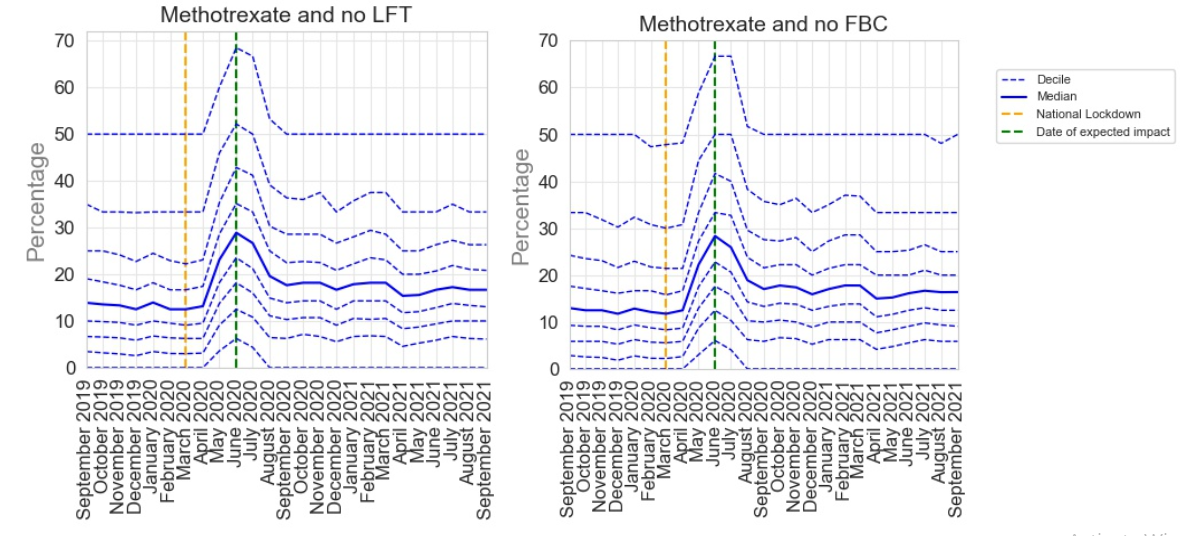
Despite its efficacy, methotrexate can have some side effects, including liver toxicity and bone marrow suppression. It is therefore important for patients prescribed methotrexate to undergo regular blood tests. NICE recommends that patients taking methotrexate should have a full blood count (FBC), liver function tests (LFT) and renal function checked at least every 12 weeks4. The PINCER indicator for appropriate methotrexate monitoring pragmatically identifies two of these key requirements (FBC and LFT):
Patients receiving methotrexate for at least 3 months who have not had a recorded:
- Full blood count (FBC) within the previous 3 months (Methotrexate and no FBC)
- Liver function test (LFT) within the previous 3 months (Methotrexate and no LFT)
During the pandemic, patients receiving immunosuppressive medications such as methotrexate were identified to be at an increased risk of contracting COVID-19. As a result these patients were advised to shield during the pandemic, which made face-to-face monitoring difficult. Considering this, it was recommended that monitoring requirements were temporarily relaxed during the pandemic, requiring monitoring every 6 months instead of 3, in patients where this was appropriate.
The results from our analysis for this indicator are shown below. Following the onset of COVID-19 in March 2020 there was an increase in delayed monitoring for both LFT and FBC. This reached a peak by June 2020, 3 months after the onset of the pandemic before sharply recovering by August 2020. This peak is likely a reflection of this temporary extension of monitoring but shows that monitoring returned to pre-pandemic levels quickly.
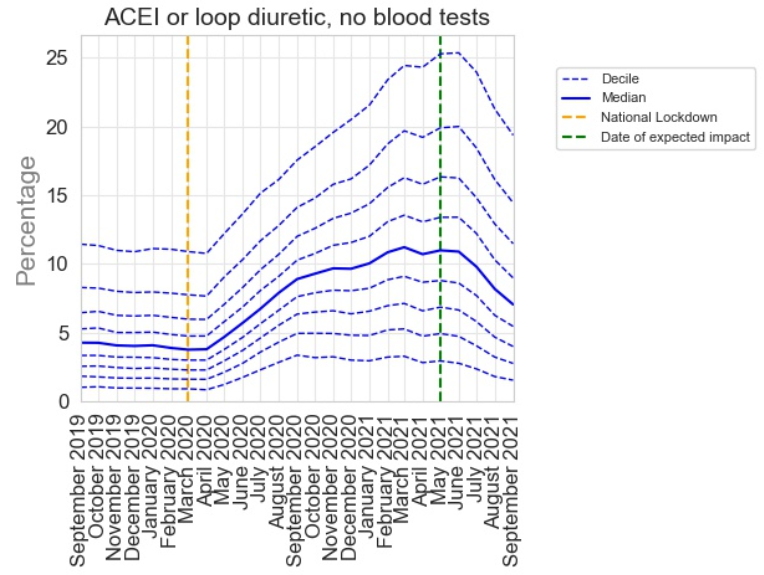
Not all of the PINCER indicators showed such a rapid recovery. For example the indicator below, which shows the percentage of patients receiving an angiotensin converting enzyme inhibitor (used for blood pressure management) without a recent blood test, had a much more protracted recovery. We think this reflects the lower risk of missed monitoring for this medication and highlights sensible prioritisation that occurred during the COVID-19 recovery period.
The impact of missed monitoring and future opportunities
The post-pandemic period provides an opportunity to push health systems to be more resilient, responsive and sustainable, including with ongoing monitoring of patients’ conditions 5. Outcomes can be assessed to identify any clinical impacts on patients or tests that can be safely delayed without unintended impacts to free up health care capacity. An audit assessing the effect of the temporary delay in methotrexate monitoring found no evidence of excess harm 6. This could suggest that delayed monitoring may be suitable in a subset of patients. However, it is important to consider the potential for confounding by patient-specific factors that can impact clinical risk. For example, patients at higher risk may be more inclined to attend monitoring.
-
Elliott RA, Camacho E, Jankovic D, Sculpher MJ, Faria R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2021 Feb;30(2):96–105. ↩︎
-
Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny MP, Sheikh A. Medication Without Harm: WHO’s Third Global Patient Safety Challenge. The Lancet. 2017 Apr;389(10080):1680–1. ↩︎
-
Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden M, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. The Lancet. 2012 Apr;379(9823):1310–9. ↩︎
-
Durski KN, Osterholm M, Majumdar SS, Nilles E, Bausch DG, Atun R. Shifting the paradigm: using disease outbreaks to build resilient health systems. BMJ Glob Health. 2020 May;5(5):e002499. ↩︎
-
Shadeed A, Kattach L, Sam S, Flora K, Farah Z. Examining the safety of relaxed drug monitoring for methotrexate in response to the COVID-19 pandemic. Rheumatol Adv Pract. 2022 Sep 6;6(3):rkac100. ↩︎

