Incidence and management of inflammatory arthritis in England before and during the COVID-19 pandemic
-
Authors:
Mark Russell
-
Posted:
- Categories:

This article is part of a series: Guest blogs
- Conducting Research Using OpenSAFELY: My Experience of the Co-pilot Service
- High Dose Dexamethasone
- How I use OpenPrescribing in my practice as a GP
- Conducting Research Using OpenSAFELY: My Experience of the Co-pilot Service
- Using electronic health records and open science in the COVID-19 pandemic
- Exploring the Impact of COVID-19 on common infections: Treatment Pathways, Antibiotic Prescribing, and Exposure
- Incidence and management of inflammatory arthritis in England before and during the COVID-19 pandemic
- Updates of OpenSAFELY Research on COVID-19 Therapeutics
- Understanding Repeat Antibiotic Prescribing in the Pandemic: Insights On Health Inequalities
- Trends in inequalities in avoidable hospitalisations across the COVID-19 pandemic
In this guest blog, Mark Russell describes the research that he has undertaken with the help of his team from King’s College London using OpenSAFELY.
Summary
Our research team at King’s College London have been using OpenSAFELY to understand how the COVID-19 pandemic has impacted on care for people with inflammatory arthritis in England. Our goal is to use routinely captured health data to transform how we monitor the quality of care for people with chronic diseases in the NHS. Our findings were recently published in two articles in The Lancet Rheumatology (here and here).
Why did we do this study?
Inflammatory arthritis conditions, such as rheumatoid arthritis and gout, have a huge impact on quality of life for patients with these diseases. Permanent joint damage and disability can occur if diagnosis or treatment is delayed. Fortunately, there are excellent treatments available to reduce symptoms and prevent complications; however, previous studies have shown that many patients do not receive the treatment they need in a timely manner.
To improve care for patients, national audits (e.g. the National Early Inflammatory Arthritis Audit) have been established to monitor care quality for people with inflammatory arthritis in England and Wales. Currently, these audits need manual data entry by clinicians, which is time-consuming and costly to deliver. Additionally, there were pauses in data collection during the COVID-19 pandemic, which limits our ability to monitor care for patients.
Our aim was to use routinely collected health data, via the OpenSAFELY platform, to transform how we monitor chronic health conditions in the NHS.
How did we do this study?
We analysed GP and hospital data for 24 million people in England via the OpenSAFELY platform, to evaluate care quality for people with new diagnoses of inflammatory arthritis. For autoimmune types of inflammatory arthritis, such as rheumatoid arthritis, we replicated quality standards from the National Early Inflammatory Arthritis Audit, including the time to assessment by a hospital specialist, and the time to prescription of an disease-modifying treatment (e.g. methotrexate). For gout, we replicated quality standards recommended by the National Institute for Health and Care Excellence (NICE), including the proportion of patients who are prescribed a urate-lowering drug (e.g. allopurinol). We compared performance against these standards before and after the onset of the COVID-19 pandemic, at both national and regional levels.
What did we find?
We showed that the number of new diagnoses of inflammatory arthritis dropped markedly during the first year of the COVID-19 pandemic – by 20% for rheumatoid arthritis, and 30% for gout. Diagnoses increased after the first year of pandemic, but have still not rebounded above pre-pandemic levels. This suggests that many patients remain undiagnosed as a consequence of the pandemic.
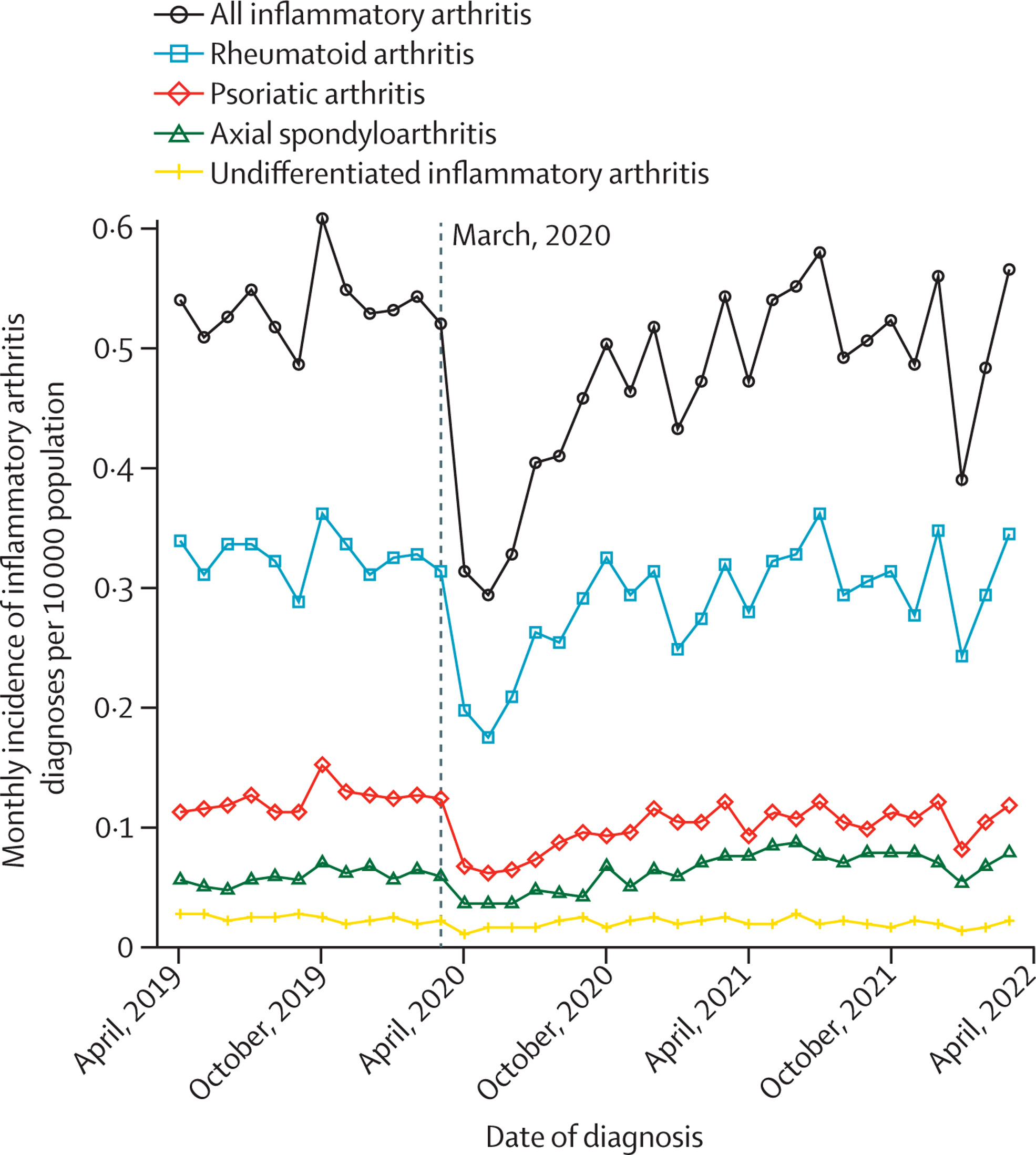
Interestingly, for patients who were diagnosed with rheumatoid arthritis or gout during the pandemic, the quality of care was similar or slightly better than before the pandemic. For example, the time taken to see a hospital specialist improved for patients with rheumatoid arthritis, while the proportion of patients with gout who were prescribed a urate-lowering drug increased. This highlights the remarkable adaptation of our health service in the face of unprecedented pressures during the pandemic.
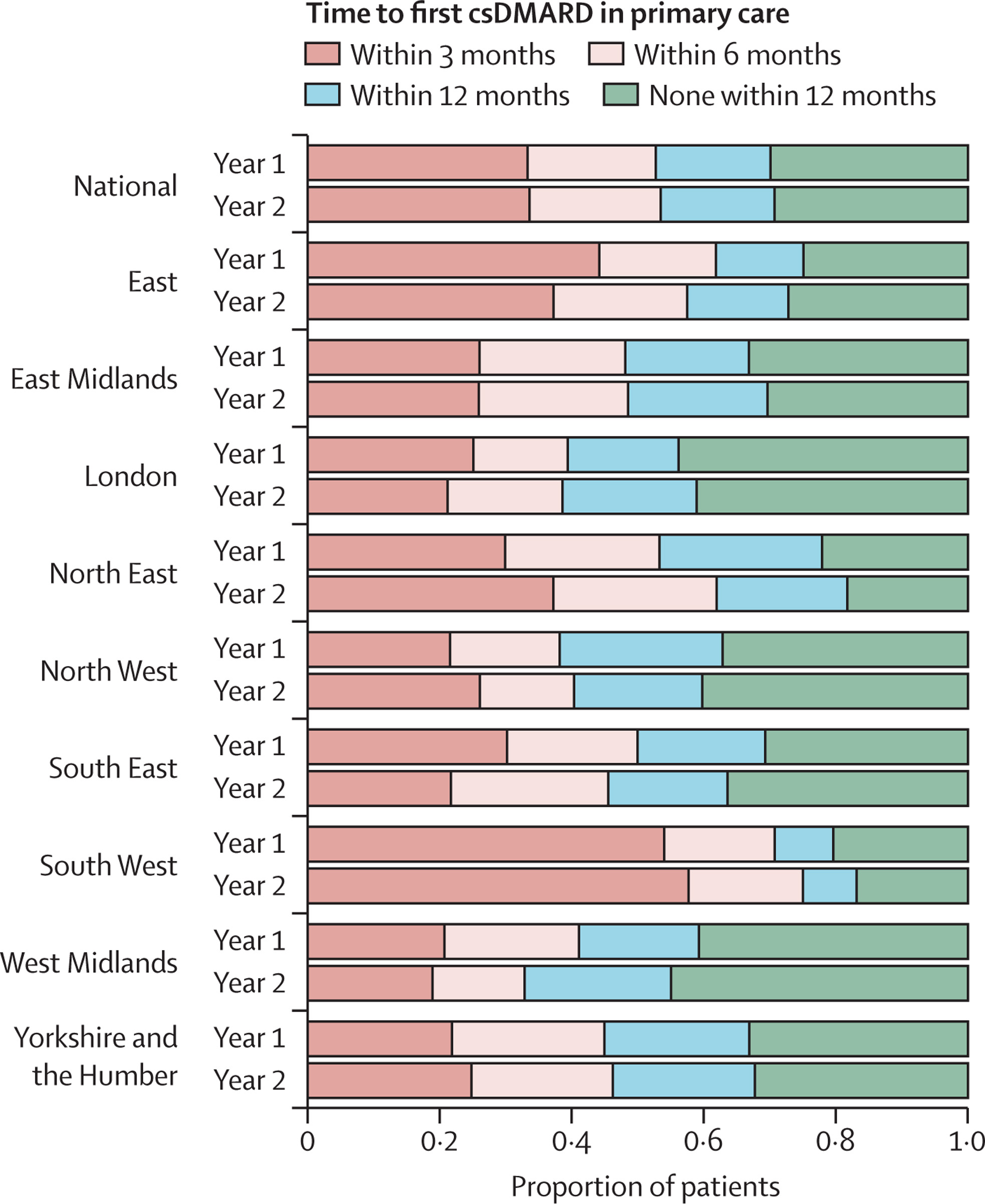
Despite this, improvements are still needed. We showed that only one-third of patients with gout are prescribed a urate-lowering drug within a year of diagnosis, whereas guidelines suggest that these medications should be offered to all patients after diagnosis. For rheumatoid arthritis, we showed that there is marked regional variation in the time to prescription of a disease-modifying medication in primary care. This highlights the need for strategies to ensure all patients with these conditions are offered the best standard of care.
Conclusions
Using OpenSAFELY, we were able to show large reductions in new inflammatory arthritis diagnoses during the COVID-19 pandemic. For patients who sought medical attention during the pandemic, the impact on care quality was less marked than might have been expected, and recovery was swift. Perhaps the most important message of our work, however, is that it is possible to use routinely collected health data via OpenSAFELY to transform how we monitor long-term conditions in the NHS.
Next steps
Our next goal is to use what we have developed to improve care for patients. We plan to incorporate our analysis code into the National Early Inflammatory Arthritis Audit, to enable near real-time monitoring of care at local, regional and national levels. We have developed an OpenSAFELY report to facilitate this. We hope to expand our work to other chronic health conditions. We are also working with the team at OpenSAFELY to incorporate data on high-cost drugs, so that we can evaluate prescribing of these highly effective medications throughout England.
Get in touch
All of the code used for our analyses is open and reusable (here and here) so that other researchers can adapt these methods for their own work. If you have more questions about any of our research, please get in touch via mark.russell@kcl.ac.uk.

