What is the dm+d? The NHS Dictionary of Medicines and Devices
- Authors:
-
Posted:
- Categories:

This article is part of a series: Clinical Codes
- Prescribing Data: BNF Codes
- What is the dm+d? The NHS Dictionary of Medicines and Devices
- Difference between BNF, dm+d and SNOMED CT codes
- An Introduction to Clinical Codes and Terminology Systems
- What Are Codelists and How Are They Constructed?
Over the past year we have been increasingly using NHS Dictionary of Medicines and Devices (dm+d). This blog post sets out to describe dm+d for the benefit of the wider prescribing analytics community and others.
What is the NHS Dictionary of Medicines and Devices (dm+d)?
dm+d is the standard dictionary for the medicines and devices used across the NHS. It contains standardised codes, descriptions, and metadata (such as price and pack size) for every entry. At last count it contained over 150,000 packs of medicines and devices.
Why is it important to have a standard NHS Dictionary of Medicines and Devices?
These codes and descriptions are very important when dealing with large amounts of data, to allow computers to operate consistently. All computer systems in primary care now communicate by exchanging dm+d codes with each other. For example, when your doctor generates a prescription on their computer, they select the items you need from the dm+d. The dm+d code and quantity is transferred securely over the NHS “Spine”, which delivers it to your pharmacy computer for dispensing. Last month over 62 million prescriptions were sent through the Spine, all facilitated by dm+d.
By contrast, a large hospital may have anywhere between 50 and 100 clinical software systems, each using a different coding system, which means they cannot talk or transfer information easily between them. This is one reason why your GP can’t see what medicines you have been prescribed on a hospital ward. Fortunately, the NHS has now mandated the use of dm+d. All NHS systems will soon be using dm+d, facilitating the transfer of medicines data between software and computers across the entire NHS.
What are the uses of NHS dm+d?
The stated primary use of the NHS dm+d is to improve ‘Interoperability’, which means computers talking to each other in the manner described above — but there are lots of other uses. At OpenPrescribing, we have linked prescribing data to dm+d, which means we can make use of the richer data that it provides. We previously described a simple but really useful feature of the dm+d, allowing us to show user-friendly names of medicines on OpenPrescribing. The dm+d makes more advanced filtering possible, such as listing all medicines delivered by injection, or all drugs not allowed to be prescribed in primary care. We have already started to implement new analyses utilising this structured data, such as our recent herbal measure.
Summary level descriptions of most important concepts
There are a bewildering number of data fields in NHS dm+d; we describe the most important ones here.
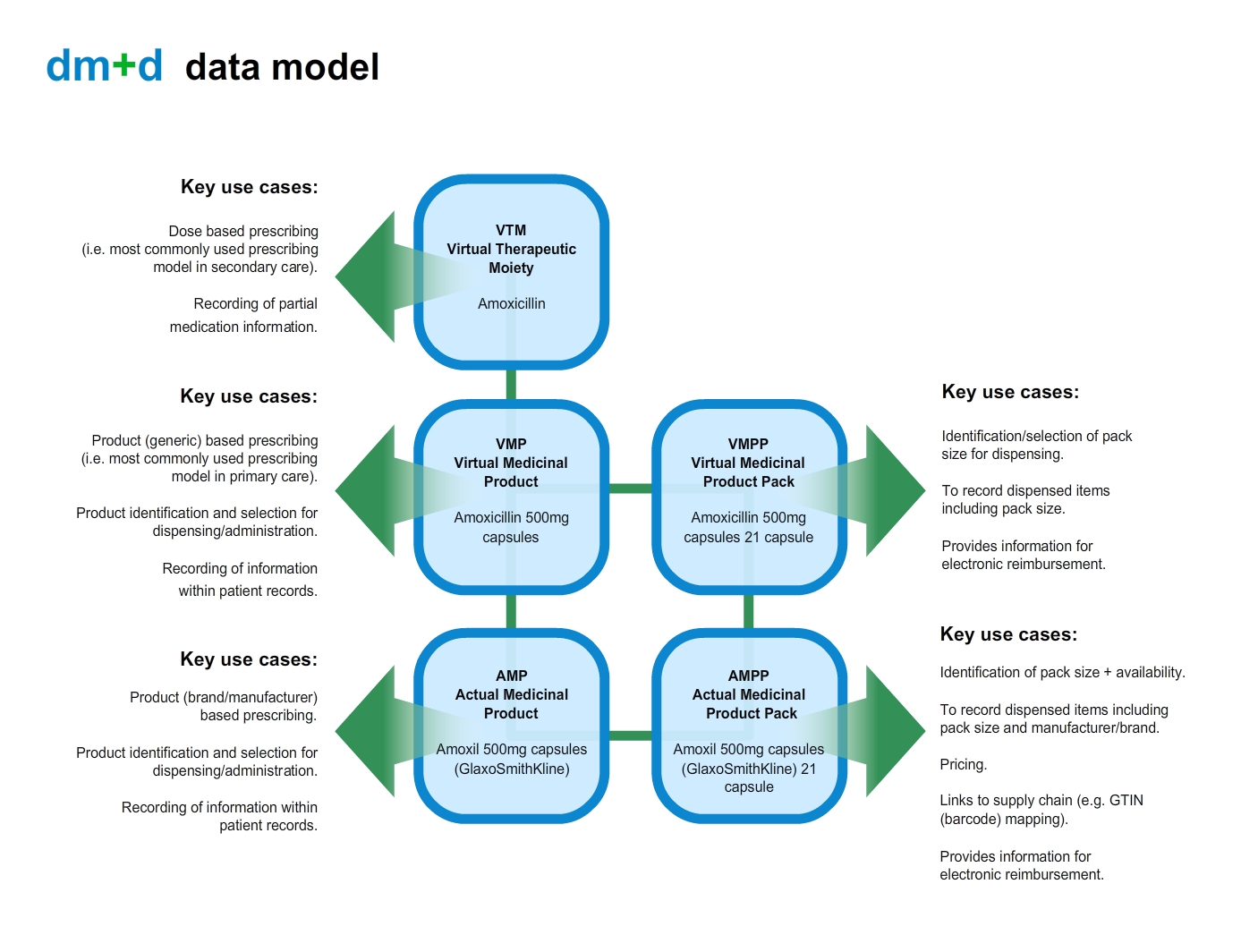
Virtual v Actual
A description starting with “actual” is a real product description: it describes a medicine or device that has been made available by a specific manufacturer or supplier, much like when a doctor prescribes by brand.
A description starting with “virtual” is an abstract description: it describes a general class of medicine or device that may be available as an “actual” product, much like when a doctor prescribes generically.
 Source: NHS Business Services Authority
Source: NHS Business Services Authority
Virtual Therapeutic Moiety (abstract) — VTM
Example: Amoxicillin
The VTM is a highest level within dm+d. It is an abstract representation of a medicine or device. It is just the chemical name without any mention of the strength or formulation.
Virtual Medicinal Product (abstract) — VMP
Example: amoxicillin 250mg capsules
The VMP is the first level within dm+d. It describes the abstract or generic medicinal product.
Virtual Medicinal Product Pack (abstract) — VMPP
Example: Amoxicillin 250mg capsules — 21 capsule
The VMPP is closely linked to VMP in the hierarchical model of dm+d and additionally it identifies the amount of a product that is available in a pack. This is expressed differently depending on the type of formulation (e.g. liquids, tablets, etc). for example ml in liquids, number of tablets etc.
Actual Medicinal Product (real product) — AMP
Example: Amoxicillin 250mg capsules — Actavis UK Ltd
The AMP provides a unique description of a medicine or device that has been made available by a manufacturer or supplier but does not describe the pack size or the quantity of the medicine or device.
Actual Medicinal Product Pack (real product) — AMPP
Example: Amoxicillin 250mg capsules — Actavis UK — 21capsule — 3×7 capsules
The AMPP is below AMP in the hierarchical model of dm+d and additionally it identifies the amount of a product that is available in a pack. This will be expressed differently depending on the type of formulation, for example ml in liquids, number of tablets etc.
Further reading
For more information on the NHS dm+d you can consult the full technical guidance made available by NHS Business Services Authority; in particular, the Primary Care Implementation Guidance and the dm+d Technical Specification.If you have any questions, more insight into dm+d that you can share or would like to request a topic for a future blog on the NHS dm+d please get in touch at feedback@openprescribing.net.
Get in touch
At the Bennett Institute we pride ourselves on developing our tools swiftly and iterating them based on the needs of our users. We believe that the most useful tools are built by listening to our users and pooling them with the knowledge and skills of our small mixed team of clinicians, academics, and software engineers. As always please get in touch and tell us what you are using the dm+d browser for and what improvements you would like to see.