2018 Round-Up
-
Posted:
- Categories:

In our third full year of existence we produced even more exciting outputs and continued to grow. We welcomed Lydia Berry, back from maternity leave; Dave Evans, Consultant Programmer, who joined the OpenPrescribing technical team; and Brian MacKenna, an Honorary Research Fellow Pharmacist and member of the NHS England Medicines and Diagnostics Policy Unit. We also welcomed Darren Smyth, a UK and European Patent Attorney - our work so far includes our pregabalin papers (here and here), and he has also contributed to our EUCTR work.

We’ve delivered a range of great new features on OpenPrescribing, new trial tracker sites, and a large portfolio of papers published.
OpenPrescribing.net
It’s been a very busy and productive year for OpenPrescribing.net with a whopping NINETY THOUSAND unique users this year, and masses of new people signing up to get alerts on their practice/CCG. Some of our exciting new features are summarised below.
New Low-Priority consultation measures
NHS England announced a new “Do Not Prescribe” list for consultation. Within an hour we made graphs showing every GP practice’s prescribing of these items. You can drill down to CCG level, and then practice level.
New dashboards with measure categories
A long-awaited new feature was implemented on our CCG and practice dashboards: you can now filter measures by category to view a small group of related measures together. You can also browse the categories here for all CCGs, and read more on our blog.
All England Dashboard
We launched the All England dashboard that allows anyone to monitor the quality, safety and cost effectiveness of prescribing across the whole country as a whole. We display national performance on all of our measures, and for the first time we have aggregated all the potential savings in one place, to produce some rather large numbers…

Make sure you sign up to our Newsletter to keep up to date with all the coming developments!
OpenPrescribing Events
In October we presented our OpenPrescribing work at the annual RCGP conference in Glasgow, with a workshop demonstrating OpenPrescribing.net to a room packed full with hundreds of eager GPs and other staff. Ben Goldacre gave a plenary speech you can watch [here] (slides [here]).
We are passionate about data being in the hands of the many, so it was great to see so many GPs with their eyes open wide to accessing this data for themselves.
In June we delivered a workshop on Producing Data-Driven Tools at EvidenceLive in Oxford, demonstrating examples from our work in the Bennett Institute, including OpenPrescribing.
OpenPrescribing Papers
We have published many papers this year, describing our informatics methods, practice variation, behaviour change, and policy issues.
Informatics methods
These papers describe our long-term trends tool (check out the tool here); our “price-per-unit” savings tool (find the tool on your CCG/practice pages); and the complex statistics behind our monthly practice and CCG alerts (sign up for alerts from your CCG/practice page).
Practice variation
In these papers we explore trends, geographic variation, and factors associated with prescribing behaviours in different clinical areas: homeopathic remedies, gluten-free foods, other low-priority treatments, diabetes, antibiotics and opioids (in press). You can explore the latest data on prescribing of these items by any practice and CCG using our measures or the Analyse page.
Behaviour change
In these papers we study the impact of new evidence, guidelines and policies on prescribing behaviour, including tamoxifen, antibiotics for UTIs (preprint), antibiotic reduction initiatives (in press), NHS England low-priority treatments. We also assessed how prescribing changed after practices had used OpenPrescribing and we are carrying out randomised trials to study the effect of actively contacting practices/CCGs about their prescribing.
Policy
We measured the impact on the NHS of the second medical use patent for the use of pregabalin, and discussed the implications. GPs were told to prescribe the branded form of this drug when used for neuropathic pain, resulting in excess costs to the NHS of up to £500m. This patent has now been ruled invalid by the Supreme Court, and there may be claims against Pfizer towards the excess prescription costs to the NHS.
OpenPrescribing and Policy
The Health Select Committee produced a report on Antimicrobial Resistance. The Bennett Institute submitted evidence on the variation in antimicrobial prescribing we have identified using OpenPrescribing. We were delighted that the committee found our evidence useful and made recommendations based on it (they also included lots of lovely OpenPrescribing graphs!).
We were also pleased to see one of our papers reviewed in the NICE Evidence Commentary. Our paper explored tamoxifen prescribing following a change in guidelines in 2013 allowing it to be prescribed for chemoprevention.

OpenPathology
We had a very successful first technical meeting for our new service, OpenPathology.net, modelled on our work with prescribing data. We think that good quality data tools are vitally important for better clinical care, combining cutting edge data science under the bonnet with user-friendly service. Work from our team has already shown enormous increases in the use of tests, and substantial variation. There are more details on the OpenPathology.net website if you are interested. As ever, get in touch if you’re interested to collaborate or have specific user-needs that you would like addressed.

Research Integrity
FDAAA TrialsTracker
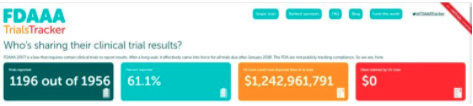
Early in the year we launched our FDAAA TrialsTracker which gives you a live look at whether individual sponsors and trialists are meeting their responsibility to report the results of clinical trials on ClinicalTrials.gov. You can read all about our methods, in detail.
It now shows that fines in excess of $1 Billion could have been collected for unreported trials. There has been no indication that the FDA has issued a single fine to date.
EU TrialsTracker
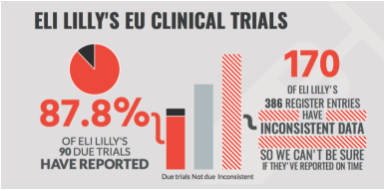
We also launched the EU TrialsTracker this Autumn, showing the results reporting status for every trial on the EU Clinical Trials Registry (EUCTR). We published in the BMJ a detailed overview of our methods, an analysis of the factors associated with non-reporting and a discussion of the data issues that make assessing compliance difficult on EUCTR.
TrialsTracker Impact
Our work on these two trackers has had tremendous media coverage including in the BBC, Reuters, Science, Wired, The Telegraph, and numerous others.
We’ve also received great, positive feedback from staff at numerous US and EU institutions and from industry, telling us that our trackers are helping them manage their trial reporting responsibilities. You can catch up with this work on our blog. The launch of the EU tracker elicited outstanding and positive responses from the EMA, House of Commons Science and Technology Committee and IQWiG, the German equivalent to NICE.

Unreported Trial of the Week
Following the launch of our FDAAA TrialsTracker, we began writing a series for the BMJ examining trials that have failed to report their results in accordance with the law. The Unreported Trial of the Week profiles a new trial each week covering its clinical significance and interesting topics related to the legal requirements to report under EU and US law. To date the series has 26 entries, many with excellent co-authors. If you’re new to the series, check out the introductory post here.
Some of these trials have reported or fixed inaccuracies in their entries, while other remain totally unreported. Look forward to more Unreported Trials of the Week in the new year, along with an overview of what the project has covered to date. If you spotted an unreported trial you would like to see highlighted, please let us know. And as always, we hope the sponsors of all unreported trials report their results soon!
Prize Winning
Our TrialsTracker work was awarded a Cochrane-REWARD Prize for Reducing Research Waste. We shared 2nd place with the wonderful James Lind Alliance; and were humbled to finish behind the mighty EQUATOR Network.

Papers
Our Retractobot project is ready for launch; with the RCT protocol in final revisions stage. Our research paper on conflicts of interest among UK NHS doctors was published.
Policy Work
Alongside all of our other work we have been putting our ideas into action in the policy community with senior civil servants, policymakers, Select Committees, and organisations such as the NHS National Information Board. Our Director Ben Goldacre was announced as Chair of the HealthTech Advisory Board reporting to Matt Hancock, Secretary of State for Health.
Onward!
All round, we think our core manifesto is proving to be a success: software engineers, clinicians, and academics, all working together to produce tools as well as academic papers. Onward!